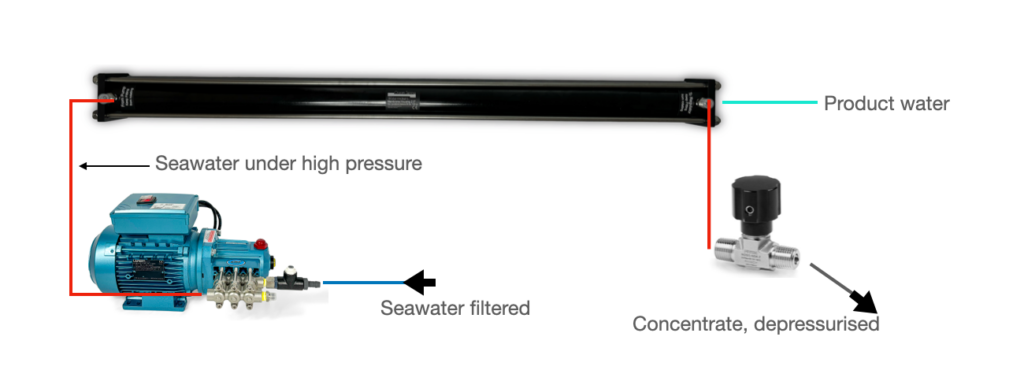
Let’s make that very short: Previously filtered seawater is pumped through the membrane under high pressure. What comes out is the desalinated product water and an even saltier wastewater – the concentrate. In order to represent this function, the basic system structure is always the same between manufacturers. The main components required are a high-pressure pump, a membrane housing with a reverse osmosis membrane and a pressure regulator where the pressure is adjusted.
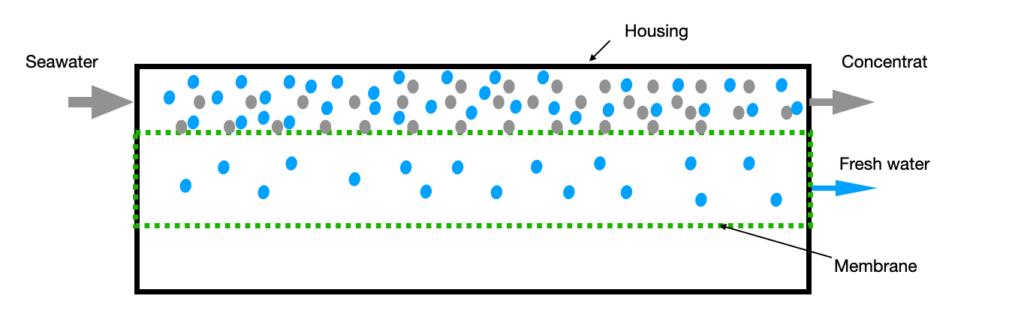
The principle is called reverse osmosis and as the name indicates, reverses the natural process of osmosis and removes fresh water from salt water. First of all, it is important to know and understand the effect of osmosis. Osmosis refers to the urge of a liquid to flow from one side of a semi-permeable membrane to the other side to compensate for differences in solute concentrations. To put it simply: If two amounts of water with different salt contents are separated by a membrane that is permeable to water but not to salt, water will flow from the less salty side to the other, saltier side.
This effect is reversed with reverse osmosis. This requires the reverse osmosis membrane and a pressure that is above the osmotic pressure of the seawater. Simplified the membrane is a very fine sieve that allows almost exclusively water molecules to pass through and retains >99% of dissolved salts, bacteria and other contamination. If such a membrane is flushed with salty water under the appropriate pressure, water will penetrate to the fresh water side but not dissolved substances such as salt.
As a result of this process, the salt concentration on the seawater side continues to rise along the membrane, as more and more water has already reached the other side and been removed from the concentrate. This effect results in a limiting factor for the possible fresh water production based on the pumped volume of sea water.
There are various factors that determine how much fresh water can be obtained. These include the membrane surface, the volume flow of seawater, its salinity, temperature and pressure.
The first two factors are firmly defined by the chosen system. The size and number of membranes determine the surface, the volume flow of seawater is defined by the pump. Both are important variables when designing the system.
The salinity of the sea water surrounding us varies depending on the body of water in which we move and determines its osmotic pressure. In the Baltic Sea, for example, with its 1-2% salt content, a pressure of around 35 bar is sufficient to produce fresh water. In the large oceans, such as the Atlantic, around 55 bar are necessary to produce fresh water at around 3.5% salinity.
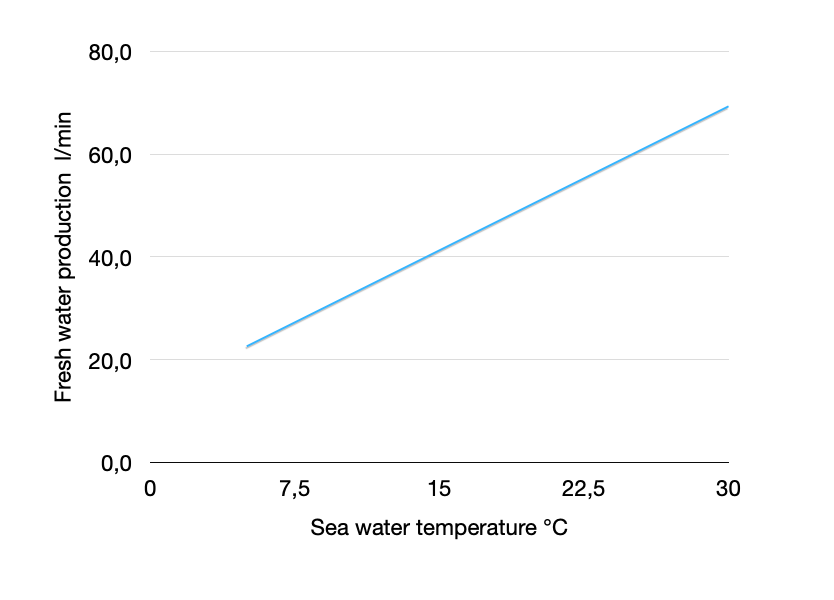
The temperature of the water also influences the osmotic pressure and thus reverse osmosis. The higher the temperature, the lower the osmotic pressure. The information about the performance of watermakers usually refers to seawater at 3.5% salinity and 25 °C. If you are traveling in colder waters, you will notice a lower fresh water rate with otherwise the same parameters. On the left is a simplified illustration to illustrate the effect for an exemplary system with 60l/h nominal fresh water production.
Continue learning more about energy efficiencies of watermakers!